RTG-ReMPro
Projects
P3 Theilig
O-GlcNAcylation and regulated proteolysis of proximal tubular LRP2/megalin linking cellular micro-and macropinocytosis with
chronic kidney disease
Objectives
Based on the previous findings, we aim to elucidate whether mTORC1 and VPS34 affect LRP2 function by altering its glycosylation status (Fig. P3, E). Additionally, other glycosyltransferases expressed in the proximal tubule and involved in LRP2 modification and mTORC1/VPS34 mediated signaling will be identified. Furthermore, reduced LRP2 glycosylation promotes shedding and RIP of LRP2 ectodomain with nuclear translocation of the C-terminus, a mechanism associated with CKD (Fig. P3, F). We aim to identify the C-terminal fragment mediated gene transcription and relate altered gene expression to CKD development.
Aim i) Determination of altered glycosylation of LRP2 by intracellular glucose/glutamine concentration and mTORC1 and VPS34 signaling
Aim ii) Determination of LRP2 C-terminus induced altered gene expression and its association with CKD
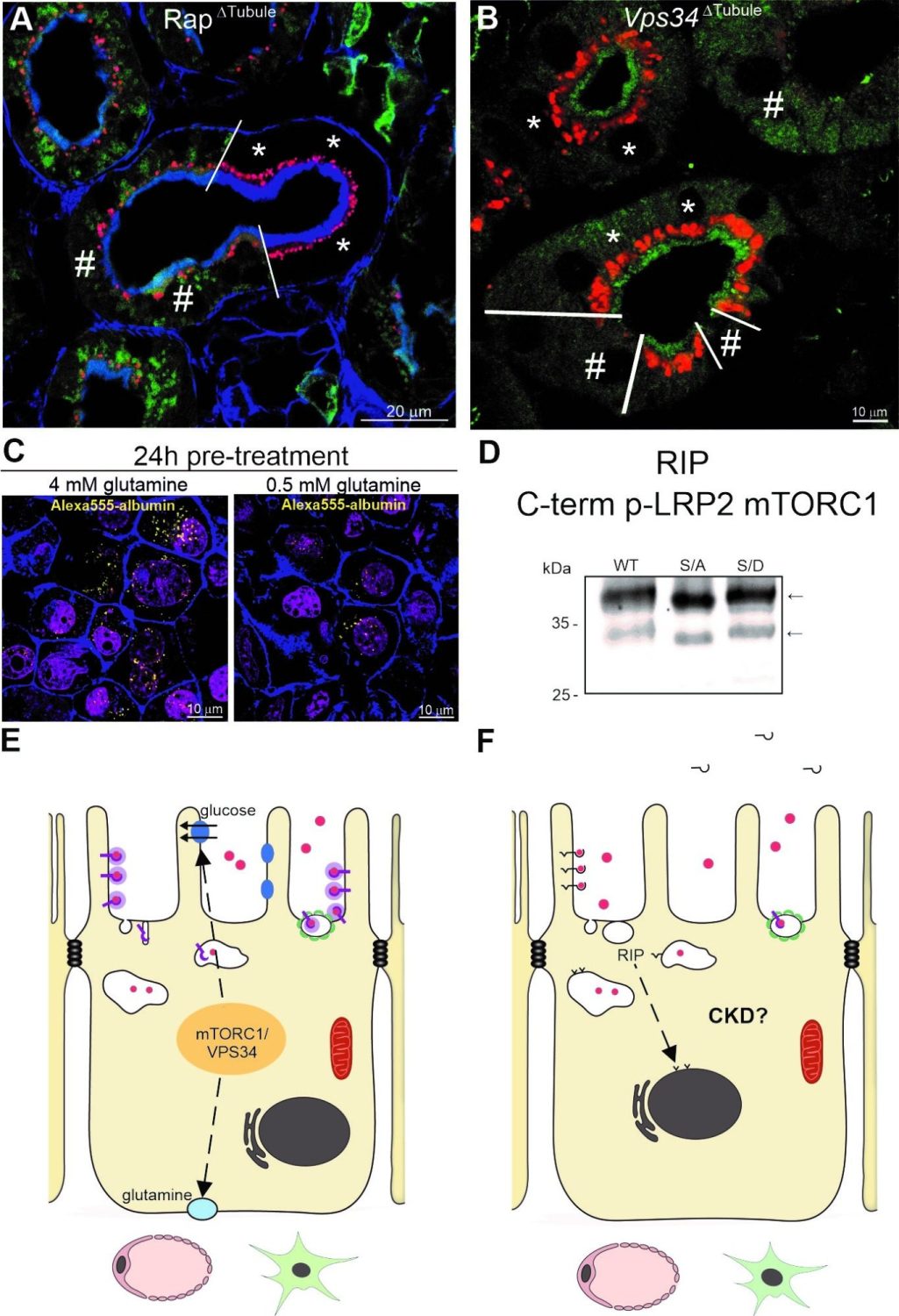
mTORC1 (A) and VPS34 (B) increase LRP2 ligand uptake (red) in proximal tubular raptor/ Vps34 depleted mice, Rap∆Tubule and Vps34∆Tubule. Wt cell are marked by an asterisk and Vps34/mTORC1 deleted cells by a hash. (C) Low intracellular glutamine concentration inhibits Alexa555-coupled albumin uptake in proximal tubule cells. (D) Immunoblot of phosphorylated C-term LRP2 mutants are processed by RIP using an antibody directed against the LRP2 C-terminus. (E) Summary of aim I hypothesis; mTORC1 and VPS34 augment intracellular glucose and glutamine content to positively regulate protein uptake by LRP2. (F) Summary of aim II; RIP of LRP2 followed by nuclear translocation of LRP2 C-terminus affect gene transcription and may lead to a decline in kidney function and CKD.

